As the Biden administration prepares to regulate hemp and CBD products, it faces a unique challenge: creating a set of rules meant to rein in an essentially rule-free, chaotic industry.
State laws and regulations exist, of course, but those are often spottily enforced, and even in the best of circumstances add to the confusion by forcing companies to adhere to a patchwork of laws to operate nationally. Fly-by-night outfits take advantage of this by essentially ignoring regulations, but that makes it even harder for the more legitimate players struggling to maintain their reputations.
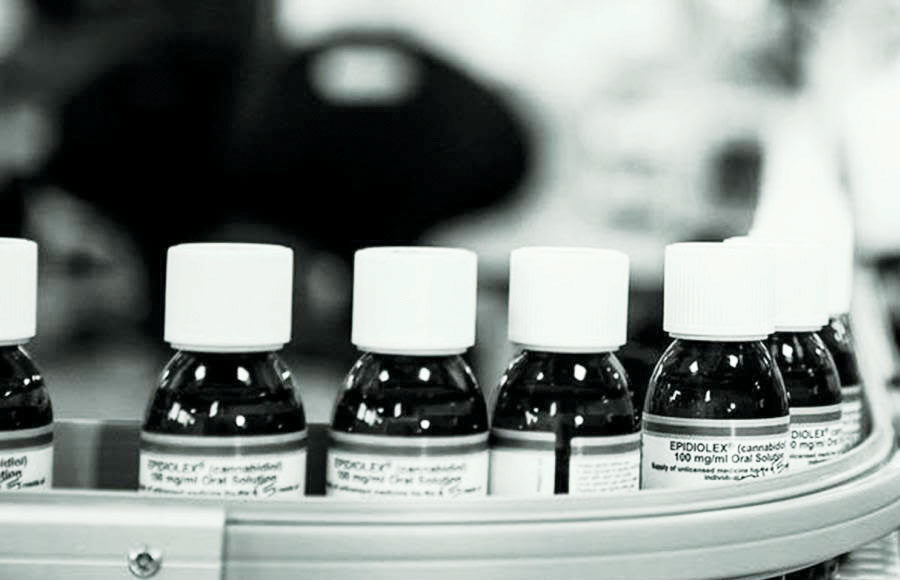
“The challenge we face now is there’s one CBD medicine with FDA approval, but thousands of companies are selling CBD containing products without FDA approval,” Brian Smith, CEO and founder of Big Sur Scientific said in an October article for Cannabis Science and Technology.
The specific medicine he was talking about was Epidiolex, which the FDA approved in 2018 as a prescription-only treatment for certain kinds of seizures. The CBD-based drug, made by GW Pharmaceuticals, went through the same lengthy, painstaking process that all pharmaceutical drugs undergo before getting the FDA’s approval.
Even so, CBD-based products claiming to be medicinal are all over the place, from both responsible companies and marginal characters making cannabis oil in their garages, sometimes with handwritten labels. Often, even the legitimate products don’t necessarily measure up to their own label claims, because the industry doesn’t really have a standardized testing regime. The illicit products might not contain any CBD at all.
Hence the need for uniform and federal regulation, according to people like Smith.
“This situation is–to the best of my knowledge–unprecedented: that an active pharmaceutical ingredient is made available to the general public before FDA approval,” he wrote. “How does the FDA go about regulating an industry that has sprinted ahead of it in terms of product development and sales?”
CBD (cannabidiol) is a component of the cannabis plant that doesn’t get you high, but has some–and possibly many–therapeutic benefits.
Aside from its proven effectiveness in treating certain kinds of seizures, CBD might be effective in treating pain, nausea, insomnia and many other ailments. It can be extracted from both marijuana and hemp (the latter plant contains no THC, the stuff in marijuana that gets you high). The lack of solid knowledge of what CBD can and cannot do stems from the fact that, thanks to cannabis being illegal for decades, scientific research is relatively scant. That presents a maddening conundrum for regulators.
One central question is whether CBD should be designated as a pharmaceutical drug or a dietary supplement.
The former designation would end a lot of confusion, but it would put many operators–both legit and sleazy–out of business. The latter would keep barriers to entry low and, assuming the existence of a decent regulatory regime, enable greater access to genuine CBD to more people at a lower cost.
The parts of the industry that want CBD designated as a dietary supplement face a challenge partly of their own making: for years, they have run around referring to cannabis–both CBD and psychoactive THC–as “medicine.”
If it really is a medicine, then, logically, it should be treated as such by the federal government. The other problem here is that the dietary supplements industry is built on a foundation of bullshit claims, thanks to the laxity of federal laws that govern it. Some supplements are effective, many aren’t. Consumers are left basically on their own to determine which is which.
The Biden administration will have to work through all of these challenges if it hopes to design a reasonable regulatory regime for CBD.
What should consumers do in the meantime? In California, at least, the best bet is to purchase CBD products only from licensed cannabis dispensaries rather than, say, from the counter at a shop that mainly sells sneakers and skateboards. At dispensaries, at least you can be sure the products have been tested, and be reasonably certain that what’s on the label is what’s in the product.
.Pot Shots: The Cannabis Industry Faces A Catch-22 In Future Federal CBD Regulations